BIOE 301D: Hands-on microfluidics laboratory
Microfluidic devices developed for biomedical research in academic labs often fall short of their intended biological and medical potential due to a persistent technology transfer gap between the engineers who design them and the biologists who could benefit most from their use. To address this, we have developed a new inquiry-based approach for graduate bioengineering education that closes this technology transfer gap by leveraging graduate student talent and creativity to meet the unmet needs of biological laboratories. Unlike many traditional fabrication laboratories in which devices created by students are discarded at the end of the course, this approach directs student efforts towards designing and fabricating devices desperately needed by collaborating bioscience laboratories. In the process, student experience first-hand the unexpected challenges inherent to cutting-edge research and learn to think creatively to solve them.
For more information on the Foundry, check out the Foundry website here.
Year 6: Winter 2022-2023
Teaching Team: Polly Fordyce, Hawa Thiam, Jennifer Ortiz-Cardenas, Hope Leng, Luis Santiago Mille, & Daria Wonderlick
Project #1: Microfluidic generation of single and double emulsion droplets to encapsulate large cells
Student team: Andy Hung, Annie Nguyen, Tianyu Lu, Maya Sheth
Check out Professor Hawa Thiam’s new lab website here: https://hrthiamlab.stanford.edu/
While droplet microfluidics has proven to be a powerful tool for single-cell analysis, the commercially available 10X Genomics droplet generator has limitations in accommodating large cells, as their maximum supported cell size is 30 μm in diameter. The maximum theoretical cell size as determined by the cell channel dimension is 60 μm. To address the need of single-cell sequencing and other droplet-related assays for cells beyond 60 μm in diameter, we designed and tested proof-of-concepts for microfluidic single and double emulsion droplet generators that can accommodate cells or other objects up to 250 μm in diameter. We developed a single emulsion droplet generation system that generated droplets in the range of 240 to 500 μm in diameter aimed to encapsulate both large cells and barcoded beads, making it a valuable tool for single-cell analysis. We also developed a double emulsion droplet generation system that employs a two-step process to encapsulate large cells. By controlling the flow rates of the three phases involved in the process, we were able to generate uniform double emulsion droplets with a mean inner diameter of 240 μm and mean outer diameter of 360 μm.
Co-encapsulation of double emulsion droplets (orange) and MRBLEs beads (blue) in single emulsions.
Encapsulated plant protoplasts engineered to constitutively express GFP from large Nicotiana benthamiana leaves in single emulsion droplets.
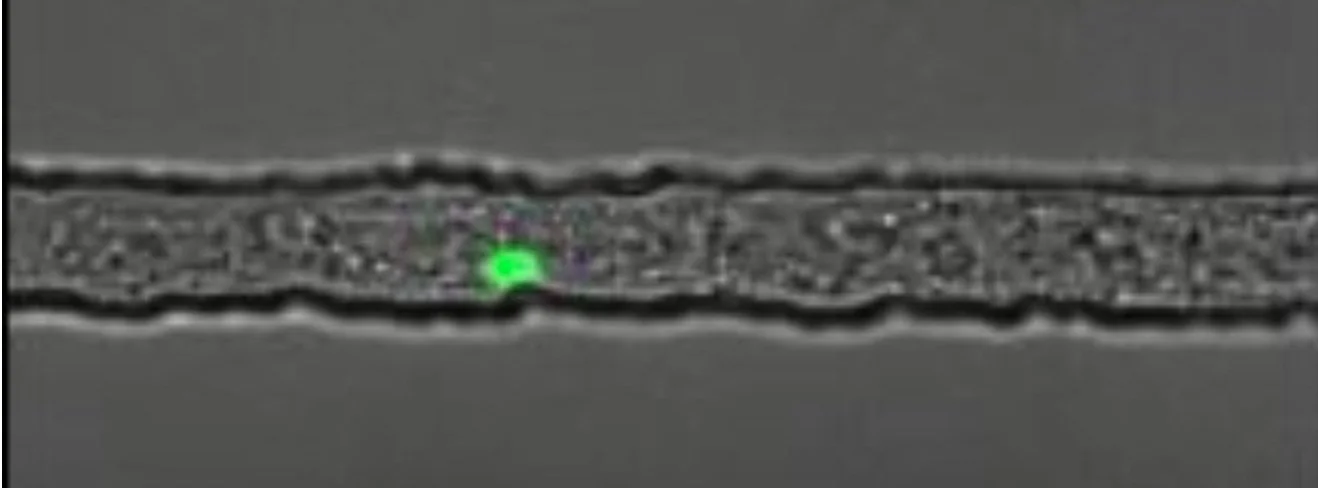
Project #2: A Microfluidic Device for Long-Term Imaging of Gut Repair in Mutant L1 C.elegans larvae
Student team: Christopher Choi, Emily Gardner, Kenna McRae, Carolina Rios-Martinez, and Heena Saqib
Collaborators: Lauren Cote (Jessica Feldman’s lab)
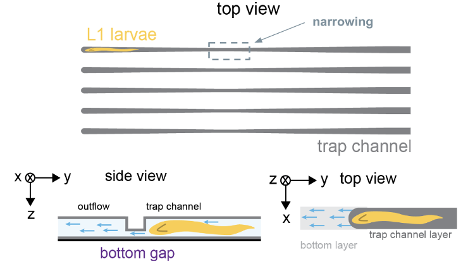
Caenorhabditis elegans (C. elegans) are free-living transparent nematodes that live in temperate soil environments. Recently, Jessica Feldman’s lab at Stanford along with the Pickett lab at SJSU have discovered an exciting model for congenital repair of damaged epithelial tubes using the C. elegans intestine. The conserved scaffold protein PAR-3 is responsible for the polarization of epithelial cells into distinct apical, lateral, and basal cortices, which is essential for epithelia to act as selective barriers. Preliminary data has shown that PAR-3 depleted C. elegans worms die before hatching from eggshells due to disconnected epithelial cell walls. Nevertheless, worms with gut specific apical PAR-3 depletion were able to repair their cystic guts through re-expression of PAR-3. We developed microfluidic devices that can trap and align 40 mutant L1 C. elegans larvae at once for long term imaging, restricting the activity of the mutant worms without the need to introduce immobilizing drugs. The trapping mechanism was optimized by implementing and testing the dimensions of a wide entrance region (fixed at 20µm), a narrow region, a trapping region, and the final taper region. We successfully performed long term imaging (72 hours) in which no worms escaped the traps and remained alive and aligned. We were also able to observe distinct repair of mutant L1 C. elegans worms at the end of the session.
Schematic of trapping mechanism.
C. elegans at end of 10 µm trapping region.
Project #3: Trapping double emulsion droplets for time-lapse imaging
Student team: Nicolai Dorka, Ishan Gaur, Douglas Henze, Jessica Karaguesian, Ernst Pulido
Collaborators: Ali Lashkaripour (Polly Fordyce’s lab)
Trapping individual single and double emulsions over time bears great potential for high-throughput screening with time-resolved information. Nonetheless, most droplet-based assays rely on end-point measurements either through on-chip sorting or off-chip FACS sorting (for double emulsion droplets). Therefore, information regarding dynamics and kinetics is often lost in droplet-based assays. To this end, the ability to image individual droplets over time enables the observation of transient responses. We developed two chip designs that enable time-resolved high-throughput screening of double emulsions: microwells and U-traps. Trapping double emulsions with microwells had several advantages over U-traps. First, microwells had a higher trapping efficiency, making them more suitable for valuable and less abundant samples. Droplet trapping in U-traps rely on collision to break the snake flowing pattern around the traps. This requires a higher droplet density compared to the microwell design. Second, double emulsion droplets remain captured in microwells with or without flow, whereas U-traps need a constant flow rate, which makes microwells the preferred choice for transport and set up changes (e.g. switching between imaging modalities).
Double emulsions trapped in U-trap device.
Double emulsions trapped in microwells device.
Project #4: Long-term Biofilm Imaging Using a Microfluidic Device
Student team: Hajime Fujita, Xiangmeng Shawn Cai, Isabel Goldaracena, Emilie Kono, Deepak Gopalan
Collaborators: Soumaya Zlitni (Ami Bhatt’s lab)
Biofilms are assemblies of microbes on a surface, embedded in an extracellular matrix that act as a protective barrier for the microbes, shielding them from the external environment. This means that bacterial biofilms are much more resistant to antibacterial treatments than planktonic bacteria and hence a common cause of persistent infections, putting a heavy burden on our healthcare system. However, most currently used assays are unable to study and quantify biofilm formation under an environment that is close to the natural physiological conditions. We developed a microfluidic device that allowed for long-term, un-disturbed bacterial biofilm formation. We fabricated different designs having different growing chambers and have the flexibility of having more replicates per experiment. Using these devices, we were able to grow bacteria (E. coli and E. faecalis) in a controlled environment (constant flow, temperature, nutrients) and observed bacterial growth and biofilm formation in the device over time.
Flow and shear stress simulations of single and dual biofilm growth chambers.
Fluorescence signal immediately after the bacteria were injected (top, t = 0 hrs) and after overnight incubation (bottom, t = 24 hrs).
YEAR 5: WINTER 2021-2022
Teaching Team: Polly Fordyce, Jennifer Ortiz-Cardenas, Ali Lashkaripour, Renee Hastings, & Eliel Akinbami
Project #1: Team Oilgae
Student Team: Kyle Denton, Mengdi He, Hope Leng, Cyrus K., & Daria Wonderlick
Collaborators: Ellen Yeh (Yeh Lab)
Botryococcus braunii secrete hydrocarbons and represent potential sources of third-generation biofuels with high oil content that do not compete with food production for arable land. B. braunii microalgae mutants with enhanced rates of hydrocarbon production and secretion could prove valuable for biofuel production, yet identifying these mutants requires new technologies capable of efficiently screening and isolating millions of algal variants based on the amount of secreted hydrocarbons. Microfluidic droplet generators can encapsulate single colonies for observation of growth and quantification of lipid production and therefore represent a promising potential approach. Here, we designed and fabricated single- (SE) and double- emulsion (DE) droplet generators that encapsulate single colonies with low multiple-cell loading events and found that 50 µm DE droplets were sufficient to encapsulate entire colonies and stain for hydrocarbon production. However, staining water-oil-water droplets with hydrophobic dyes designed to detect secreted hydrocarbons led to nonspecific staining of the oil shell that precluded effective quantification of secreted material. These devices require little technical expertise to operate and may be useful for any lab interested in elucidating new insights into cyanobacterial biology.
Encapsulating single B. braunii in water/oil droplets.
Growing B. braunii colonies within water/oil/water droplets.
BODIPY-stained water/oil/water droplets.
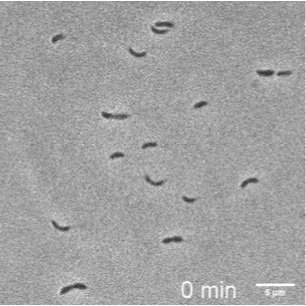
PROJECT #2: Swimmers & Stickers - A Microfluidic Cytometer for Caulobacter
Student team: Netra Unni Rajesh, Joy Doong, Linda Yang, Zi Yi Stephanie Huang, & Luis Santiago Mille
Collaborator: Saumya Saurabh (Shapiro lab)
Caulobacter crescentus is a conventional model organism for studying asymmetric cell division dynamics in response to environmental fluctuations. These organisms have two distinct phenotypes: a stalked phenotype that adheres to surfaces and a swarmer phenotype with high motility. However, separating these two cell morphologies requires multiple rounds of density gradient centrifugation in a cold room, rendering it a complex process. We designed and fabricated a microfluidic device that efficiently separates stalked cells and mobile, swarmer cells into two imaging chambers with independent media flow. The entire device fits on a microscope coverslip and also encompasses a gradient generator, enabling study of environmental fluctuation within the stalked cell chamber.
Photo showing device with generated gradient.
Composite image over 100 minutes showing stuck “sticker” caulobacter within the first chamber.
Composite image over 1.5 seconds showing highly motile “swimmer” caulobacter within the second chamber.
Project #3: Team Haystack (Sorting Muscle Stem Cells via Cross-Flow Filtration)
Student team: Kevin Chen, John Klich, Soham Sinha, Jonathan Weiss, & Jerry Yan
Collaborators: John Eugenis (Rando lab)
Despite advances in single cell sorting, isolating specific cell populations from heterogeneous samples of digested tissue remains a prohibitively expensive and time intensive process. Sorting cell suspensions by cell type is required for both fundamental research and therapeutic translation, including isolation of muscle stem cells for regenerative medicine. Here, we report on the development of a cross-flow filtration microfluidic device capable of separating particles in different size regimes (<10 μm vs. >10 μm) in a bead-based model system. In an iterative approach, we demonstrate that sorting efficiency is a function of interpillar spacing and pillar angle, and propose alternative strategies for future work.
Photomask designs for design iteration Round 1 and 2.
Image of fabricated wafer with 15 µm separations between pillars.
Image of microscope with fabricated device and tubing.
Project #4: Team Killifish (Size-based sorting of Killifish oocytes)
Student team: Ziad Ali, Ananya Chadha, Vincent Cornelius, Danielle Klinger, & Cassandra Villicana
Collaborators: Jingxun Chen (Brunet lab)
Killifish oocytes are commonly used to study aging. Given that oocytes grow with time, cells of certain ages can be selected for based on size. However, extracting oocytes within the size range of interest (50 - 200 µm) without collecting unwanted smaller or larger cells is difficult using existing methods. To address this challenge, we designed and fabricated a pinched cross- flow microfluidic device intended to filter cells outside of the target size range. The device improved the enrichment of cells within the target size range by approximately 2.5x.
Round 1: Main channel of cross-flow filtration device showing a large target cell remaining in the channel while a smaller cell gets pushed through pillars and lysed.
Round 2: Target cells persisted in the main channel of the cross-flow filtration device without lysis after the between-pillar distance was reduced to 30 µm.
YEAR 4: WINTER 2019-2020
Teaching team: Polly Fordyce, Nicole DelRosso, Louai Labanieh, & Zach Sexton
Project #1: Placenta-on-a-chip
Student Team: Anthony Agbay, Ashwin Ramachandran, Carly Weber-Levine, Mustafa Fattah, Vandon Duong
Collaborator: Jay Sarkar (Sebastiano lab)
The placenta plays an integral role in the exchange of endogenous and exogenous materials between the mother and the fetus. We sought to use this filtering function of the placenta in the application of aging by designing a placenta-on-a-chip (POC) that can be used to filter plasma. Through two design iterations, we use soft lithography to develop a two layered microfluidic device that is separated by a polycarbonate membrane. We demonstrate that our device is successfully able to flow fluid and cells. Overall, in future iterations of the POC we hope to obtain a fully functioning microfluidic filtration device that can additionally separate plasma from cells in blood.
Bonded glass device surrounding a thin membrane to facilitate co-culture.
Human umbilical vein cells (HUVECs) growing on one side of the filter cell membrane within the glass device.
Project #2: High-throughput measurement of red blood cell deformability
Student team: eliel akinbami, manish ayushman, jason hermann, Eerik Kaseniit, & sauradeep sinha
Collaborator: emily ebel (egan lab)
Our team's goal was to measure certain physical properties of red blood cells (RBCs), like their “squishiness,” since these properties are important in susceptibility to parasite infection. In particular, our collaborators were interested in comparing the susceptibility to infection by the malaria parasite against the deformability of red blood cells for samples derived from patients with different genotypes. To address this challenge with microfluidics, we designed two types of constricted channels, based on existing literature. As RBCs flow through the constrictions, they are deformed. The deformabilities of the cells therefore determine which paths the cells take. One design utilizes an array of wedge constrictions and channels of various dimensions. In our second design, we explored a branching tube configuration. One major challenge of this fabrication was carefully calibrating the doses required to achieve feature resolution of nearly one micron. A second challenge was the need for two-level fabrication to form bypass channels that were much larger than the constricted channels. In order to accomplish this, we used a multi-mask process, in which one mask defines the constricted channels, and another mask, aligned to the first using specialized markings, defines the large bypass channels. With the aid of the course staff, we were able to push the limits of these chips in terms of the small feature sizes on multiple layers. We measured our devices using a flow control system, by pulsing the RBC flow through the microfluidic device, and ultimately succeeded in observing RBCs transiting the constrictions.
Image of red blood cells passing through small tapered channels to assess deformability.
Movie showing flow of red blood cells through tapered channels.
Project #3: Team Wormz (immobilization for long-term imaging)
Student team: Nora Enright, Akshay Maheshwari, Emily Meany, & Cameron Park
Collaborator: James Ferguson (Feldman lab)
C. elegans is a small transparent nematode commonly used as a model organism. Its transparency throughout its lifespan enables live confocal imaging, offering important insights into cell migration and development. Cell migration is an important biological process implicated in wound healing, embryonic development, and cancer metastasis. There are still many unanswered questions surrounding how migrating cells sense their environment and know which direction to move in. Because of their rapid development, C. elegans can offer important insights into these migratory patterns over only several hours of imaging, in contrast to days or months for slower developing organisms. Previous microfluidic devices have successfully trapped and immobilized adult or stage L3-L4 worms for imaging, but none have been used with stage L1-L2 worms. We developed a microfluidic device to immobilize C. elegans in these earlier larval stages, optimizing our tapered channels to accommodate their small size. We successfully loaded worms in many of our devices using a syringe pump and, after testing multiple taper gradients, determined that a 15 μm tall device with a 20 μm inlet and 5 μm outlet performed best for immobilizing the target worm population. Confocal microscopy imaging revealed successful worm immobilization over 8 hours, facilitating tracking of cell migration patterns in stage L2 C. elegans by our collaborators in the Feldman lab.
Example design for tapered channel trap devices.
L2 worm immobilized in a 15 µm tall channel tapered from 20 µm to 5µm with an 0.5 mm extension.
L2 worm immobilized to allow tracking of fluorescently-labeled sex myoblast cells over 8 hours.
Project #4: Immobilizing single mammalian cells for long-term culture and imaging
Student team: Namrata Anand, Gauen Kim, Taylor Merkel, Callan Monette, Taylor Nguyen, & Peter Suzuki
Collaborator: Tahei Fujimori (Bintu lab)
Chromatin regulation plays an essential role in development, aging, and disease. Previous work in the Bintu lab has highlighted the importance of studying chromatin regulatory dynamics at single-cell resolution, which can be done through live-cell imaging. While live single-cell imaging is common in adherent cells, we are still quite limited in our ability to perform single-cell tracking of suspension cells over a long period of time. Furthermore, trapping of suspension cells presents the opportunity to release cells from their traps for collection and downstream analysis. We designed and fabricated two types of single-layer microfluidic devices capable of trapping and immobilizing hundreds of single cells over multi-day imaging experiments: a plinko-style lateral trapping device and a serpentine capture device. We successfully achieved a high capture efficiency for both devices, and incorporated two additional modular devices capable of filtering debris that may be introduced during media flow and enabling switching between cell suspension and media flow with minimal introduction of bubbles. In the future, parameters such as gap width and length could be optimized to avoid cell escape over time. Further changes, such as using multiple syringe pumps in a given experiment rather than swapping a syringe mid-experiment, are needed to enable longer-term culture without introduction of bubbles or dislodging of cells.
Move showing trapping of individual eGFP-labeled mammalian cells.
Movie showing trapping of individual eGFP-labeled mammalian cells.
YEAR 3: WINTER 2018-2019
Teaching Team: Polly Fordyce, Loza Tadesse, Peipei Lyu
Project #1: Shear-free oligodendrocyte culture with media exchange
STUDENT TEAM: Beatriz atsavapranee, patrick brennock, nicole delrosso, eva gonzalez diaz, & connor ludwig
collaborators: manasi iyer in brad zuchero’s lab
Myelination is essential for proper brain function, and dysfunction in the process is linked to several neurological disorders. Oligodendrocytes are glial cells that myelinate neuronal axons in the local environment and contribute to brain plasticity. These cells and their myelination dynamics are difficult to study due to their shear sensitivity, precise imaging requirements, and limited throughput. To address this, we designed, fabricated, and tested microfluidic devices to culture oligodendrocyte progenitor cells, differentiate oligodendrocytes, and image signaling dynamics on-chip with minimal shear stress. We successfully fabricated devices compatible with high-throughput confocal imaging, and seeded and cultured cells on-chip for up to 7 days under perfusion. In addition, we determined the maximum flow rate to reduce background calcium transient signals, and demonstrated the ability to rapidly exchange fluids on the order of seconds. These results show that microfluidic devices are a promising platform to study the impact of neuronal activity on myelination with a streamlined, on-chip workflow that minimizes shear stress.
Exciting outcome: these devices and preliminary data were used for a recent successful wu tsai neuroscience institute seed grant!
‘Horseshoe device’ cell loading: Day 1.
Loading cells into ‘horseshoe devices’.
Project #2: a hydrodynamic breadboard
Student team: michaela hinks, prima dewi sinawang, zachary sexton, ana uriarte, sasha zemsky
collaborators: endre mossige and chunzi liu in Gerry fuller’s lab
Sorting cells represents a critical need in cellular and molecular biotechnology, medicine, and tissue engineering. Although technologies such as fluorescence activated cell sorting (FACS), magnetophoresis, and optical tweezers have been widely implemented for cell sorting, these methods also require cell labeling, extensive hardware, and may only be efficient at sorting cells for short periods or in small batches. Furthermore, when sorting cells based on size selection, these methods may not be the most effective approach. Traditionally, sorting cells based on size has been a passive process involving filtration, physical force, or tailored surface chemistry methods to direct cell separation. While microfluidic devices have leveraged these passive physical properties to overcome the gap in sorting accessibility, these devices lack modularity and universal applicability without extensive design modification. Here, we present a device capable of creating a hydrodynamic pillar in which the diameter of the pillar can be adjusted by simply varying flow rates of the source and sink of the microfluidic device. Furthermore, no clogging is observed along the boundary of this hydrodynamic dipole pillar, making it possible to use deterministic lateral displacement to sort particles and cells with a wide working range. Our results show that this preliminary device is able to sort particles with diameters of 5 μm and 10 μm.
Pneumatic and imaging setup for hydrodynamic breadboard.
Hydrodynamic barrier (red) in the midst of cross-flow (blue).
Differential flow of fluorescent particles around the hydrodynamic barrier.
Project #3: high-throughput production of artificial cells
student team: Amy Bour, Suzanne Calhoun, John Eugenis, Yuxi KE, Alice Stanton
collaborators: akshay maheshwari in drew endy’s lab
Liposomes are aqueous vesicles surrounded by a lipid bilayer that are broadly used in basic science research, drug delivery, and other applications. Liposomes are typically created in bulk by mixing aqueous and oil solutions, but this approach is time- and reagent-intensive and produces polydisperse emulsions. Here, we harness microfluidics to create a droplet generator that can produce liposomes with more controllable properties and using smaller volumes of reagents. Hydrodynamic flow focusing directs multiple streams to create concentric liposome layers in a microfluidic device. We report the creation of small, cell-sized (2.5-30um) unilamellar liposomes for synthetic biology applications using a device that can be used to form double emulsions enclosing various cell components. This can enable future work creating artificial cells and further our understanding of the role of various cellular components comprising living cells. The tunability of liposome properties, small achievable liposome sizes, and small reagent volumes make this device broadly attractive for a wide variety of uses in basic science research and other delivery applications.
Image of small (5 µm) features at device nozzle.
Polydisperse double emulsion droplets with sizes ranging from 2 µm to 30 µm.
Production of small single emulsion droplets.
Project #4: a simplified device for collagen fiber production
student team: Ethan Li, abhijit lavania, jennifer Moy, elizabeth botbol ponte, grace zhong
collaborators: ada undieh in gerry fuller’s lab
Collagen is an indispensable structural protein in vertebrate extracellular matrix and is the material of choice for many biomedical applications due to its superior physical properties and biocompatibility. In particular, aligned collagen is of great interest in engineering scaffolds for tissue regeneration. However, current fiber extrusion methods are not tunable, have a large lower diameter limit, and produce fibers with inferior mechanical properties. Here, we describe the design and fabrication of various single- and multi-layer devices for collagen extrusion. We explore the effect on fiber formation from factors such as priming sequence, junction geometry, channel length, single-vs. multi-layer designs, and flow rates. We further present an optimized priming sequence which enables successful fiber extrusion.
Bright field image of 2 layer flow-focusing device.
Bright field image showing co-axial flows.
Successful formation of collagen fibers at device outlets!
Collagen fiber formation within device channels.
YEAR 2: WINTER 2017-2018
Teaching Team: Polly Fordyce, Siavash Ahrar, Yuan (soso) Xue, Alec Tarashansky
Project #1: A planarian guillotine
Student TEAM: Bauer LeSavage, Jack Silberstein, Jon Calles, and Suhas Rao
CollaboratoRS: Sam Bray and Alec Tarashansky in bo Wang’s lab
The planarian flatworm S. mediterranea is a unique model of development that can regenerate entire organisms from fragments as small as 1/279th of the original host. This extreme regenerative capacity is thought to result from the planarian’s high stem cell content (up to 30% of total cells). Chimeric worms created by fusing fragments from different worm species provide a powerful tool to study the collective action and coordination of these stem cells in regenerating an entire organism. This competition is best studied by bisecting worms along their longitudinal axis and fusing halves from opposite strains together; however, this requires the ability to consistently and efficiently bisect worms with a throughput and precision not possible via current methods. To address this, we developed a microfluidic guillotine to quickly and consistently bisect planaria and recover the halves for further regeneration studies. Our device consists of an 18 mm long, 175 µm tall, and 700-900 µm wide channel ending in a sharp PDMS blade (6 degree angle) such that worms are flowed down the channel, collide with the blade and are bisected into two pieces, with each half flowing out of its own outlet channel. We demonstrated that individual worms could be bisected and that bisected worms were viable and capable of future regeneration.
Figure 1. Schematic showing microfluidic planarian guillotines with varying channel widths after the guillotine blade.
Video 1. High speed video showing bisection of a single planarian using the microfluidic guillotine.
Project #2: a device for long-term culture of C. elegans
Student TEAM: Prashanth Srinivasan, Pengyang Li, Spencer Cesar
Collaborator: Lauren Booth from Anne Brunet’s lab
The free-living nematode Caenorhabditis elegans is a model organism for the study of changes during aging. Recently, several studies have reported that pheromones secreted by male worms cause reduced lifespan and accelerated aging in hermaphrodite (her ) worms. However, the identity of these pheromones and their mechanisms of lifespan reduction are unknown. Current methods for studying sexual interactions in C. elegans are laborious, time-intensive, and error-prone because they require manual separation of sexes and removal of progeny. Moreover, the low throughput of manual worm separation precludes the study of context-dependent pheromone secretions. A previous attempt to maintain sex separation using a microfluidic device relied on failure-prone screw valves and a single-channel loading strategy susceptible to cross-contamination of the sexes. Here we report the design, fabrication, and initial testing of a valve-free microfluidic device capable of maintaining male and her worm populations in chemical contact but physical isolation for probing the molecular mechanisms of pheromone-mediated lifespan reduction. The final device should also satisfy following criteria: able to keep 10-20 worms for 21 days (each sex); eggs laid can be removed periodically; worms in the device can be fed with E.coli ; and transparent enough to monitor and image the worms.
Figure 1. Device design schematic.
Figure 2. Hermaphrodite and male C. elegans worms swimming in microfluidic device.
Project #3: a valved device for co-culture of macrophage and bacteria
Student Team: Peipei Lyu, Andres Aranda-Diaz, Punnag Padhy, and Thomas Lozanoski
Collaborator: Keara Lane from Markus Covert’s lab
When a macrophage becomes infected with or phagocytoses a bacterium, (e.g. E. coli or S. typhimurium), the infected cell engages in a coordinated inflammatory program to destroy the pathogen; however, the response is not always successful, and sometimes the pathogens evade the immune system, leading to severe infections. Understanding the mechanisms that govern immunosuppression will help medical scientists develop effective vaccines and treatments for problematic infections. One hypothesis for immunosuppression is that the macrophages that phagocytose the bacterium are inhibited from relaying inflammatory signals to their neighboring immune cells. Current methods for studying bacterial infection of mammalian cells with live-cell imaging do not enable the response to infection to be separated from responses due to paracrine signaling. For example, conditioned media only captures signaling at one time point and does not lend itself to studying feedback between infected and non-infected cells. To address these issues, we microfabricated a device capable of spatially and temporally separating different cell populations so that the infection process can be imaged and the effects of its downstream signaling can be measured.
Movie 1. Pneumatic valve actuation.
Figure 1. Fluorescence image showing macrophages (green) and E. coli (red) within a chamber of the microfluidic device.
Project #4: a microfluidic droplet generator for testing polymer hydrogels
Student Team: An Ju, Loza Tadesse, Xinzi Wang
Collaborator: XINMING TONG from Fan yang’s lab
Hydrogel microspheres have been shown to enable encapsulation of cells and other biomolecules and reduce shear damage specially for injecting to an organism. Here, we investigated pre-encapsulation of mesenchymal stem cells in hydrogel microspheres using a microfluidic platform. We fabricated and tested two droplet forming designs (T-junction and flow-focusing), investigated hydrogel types including their polymerization parameter, and tested cell loading behavior of the droplets.
Figure 1. Microfluidic droplet generator connected to syringe pumps on microscope illumination stage.
Figure 2. Droplets produced via T-junction (top) and flow focusing (bottom) devices at different flow rates.
Year 1: Winter 2016-2017
Teaching Team: Polly Fordyce, Kara Brower, & Diego Oyarzun
PROJECT #1: a microfluidic device for studying toxoplasma gondii
Student TEAM: Sam Bray, cooper galvin, ali hemmatifar, deze kong, and tim schnabel
CollaboratorS: terence theisen and ian foe from john boothroyd and matt bogyo’s labs
Extravillous trophoblasts (EVT) grow out from the fetal placenta and invade the maternal uterus. While the establishment of EVT cells in the uterus is a vital stage in establishing pregnancy, the rarity of these cells has made them difficult to study. Furthermore, some EVTs differentiate into extremely large (>100 ɥm) and polyploid (>100 N) cells and we know little to nothing about the functions of these unique cells. To address this issue, we attempted to construct a microfluidic device with physical barriers for sorting and capture of large EVTs. We have engineered a single-layer microfluidic device to capture cells using physical separators at defined widths (90, 75, and 50 um). Using reverse flow, we selectively captured objects of defined diameters from individual chambers. This device will allow for the isolation of large EVTs for subsequent downstream analysis and functional studies (RNAseq, proteomics, motility studies).
Figure 1. Shear stress modeling results from COMSOL and particle image velocimetry.
Figure 2. Example images showing overnight growth of cells.
TEAM #2: Placentaur: a device for isolating very large cells from a cell mixture
Student Team: Alexander Tarashansky, Terence Theisen, Shreya Deshmukh, Nelson Hall, and Yuan Xue
Collaborator: Elisa Zhang from Julie Baker’s lab
Extravillous trophoblasts (EVT) grow out from the fetal placenta and invade the maternal uterus. While the establishment of EVT cells in the uterus is a vital stage in establishing pregnancy, the rarity of these cells has made them difficult to study. Furthermore, some EVTs differentiate into extremely large (>100 ɥm) and polyploid (>100 N) cells and we know little to nothing about the functions of these unique cells. To address this issue, we attempted to construct a microfluidic device with physical barriers for sorting and capture of large EVTs. We have engineered a single-layer microfluidic device to capture cells using physical separators at defined widths (90, 75, and 50 um). Using reverse flow, we selectively captured objects of defined diameters from individual chambers. This device will allow for the isolation of large EVTs for subsequent downstream analysis and functional studies (RNAseq, proteomics, motility studies).
FIgure 1. Device schematic. (A) Each separation chamber has different pillar spacings to capture cells of different sizes. (B) Integrated valves allow selective retrieval of particular cell sizes.
Figure 2. Image of the microfluidic device containing pillars with 50 µm gaps between them and 50 µm diameter beads successfully captured under constant pressure flow.
PROJECT #3: Traptasia: Devices to capture and study the cnidarian apitasia
Student Team: salil bhate, daniel hunt, louai labanieh, sarah lensch, and will van treuren
Collaborators: Cawa Tran and Heather Cartwright from John Pringle’s lab and the Carnegie Institute
Coral reefs are some of the most productive ecosystems on earth. Driving this productivity is a symbiosis between corals (phylum Cnidaria) and dinoflagellates (predominantly in the genus Symbiodinium) where the coral provides inorganic nutrients and the algae provides glucose or other fixed carbon. Coral bleaching, the phenomena where coral eject their symbionts when stressed, is a major concern for coral reefs around the globe. Unfortunately, coral reefs are currently threatened by systemic anthropogenic stressors, primarily ocean warming and acidification. Understanding the mechanics of coral bleaching is essential to responding to this threat. Here, we develop a microfluidic device that enables much more statistically rigorous quantification of coral biology in a model system, increasing the length of viable experiments by a factor of 3 and the number of replicates by a factor of 50.
Figure 1. Image showing array of traps with Aiptasia larvae within them.
Movie 1. Trapped Aiptasia larvae expelling symbiotic algae under stress.
This project was continued by some of the team members after the course finished, leading to a manuscript currently under review.
Please see the full bioRXiv post here!